Latest updated instructions on cosmetic import procedures
Cosmetics are one of the most popular and highly demanded imported products in Vietnam. However, to import cosmetics into Vietnam, individuals and businesses need to comply with regulations on policies, product announcements and customs procedures. In this article, HBS Vietnam will guide you in detail on the steps to import the latest updated cosmetics.
1. Cosmetic import policy
According to Circular No. 06/2011/TT-BYT, cosmetics are defined as:
“A cosmetic product is a substance or preparation intended for use in contact with the external parts of the human body (skin, hair system, fingernails, toenails, lips and external genitalia) or teeth and oral mucosa with the main purpose of cleaning, perfuming, changing appearance, form, adjusting body odor, protecting the body or keeping the body in good condition.
Cosmetics are not on the list of goods banned from import into Vietnam, so individuals and businesses can absolutely import cosmetics like normal goods.
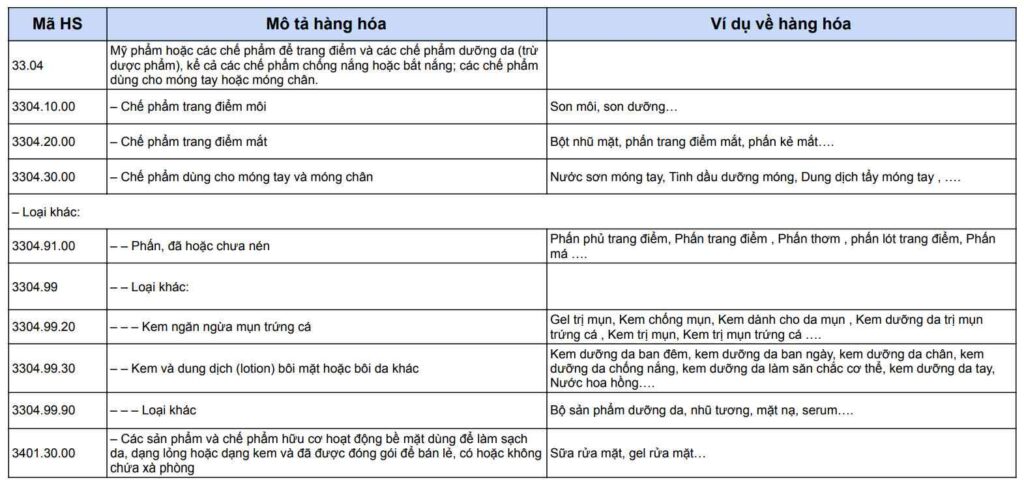
Imported cosmetic products belong to subgroup 3304, the HS code of cosmetics is determined as follows:
2. Announcement of cosmetic products
Cosmetic product announcement is a mandatory legal procedure for each individual and business importing cosmetics. Cosmetic announcement dossier includes:
- Declaration of imported cosmetics
- Certificate of business registration
- Certificate of registration for free circulation of products in foreign markets (Certificate of Free Sale - CFS) which requires:
- CFS is still legally valid at the time of licensing.
- CFS is issued by the competent authority of the country of origin or exporting country.
- CFS is notarized and confirmed by the Vietnamese Embassy or Consulate in the country of origin or exporting country.
- Copy of the enterprise's import license
- Copy of economic contract with supplier
- Copy of commercial invoice
- Copy of bill of lading
- Copy of certificate of origin (C/O)
- Copy of the product's quality inspection certificate (Certificate of Analysis - COA)
- Copy of product ingredient certificate (Ingredient List)
- Copy of product label
Applications are submitted directly to the Drug Administration of Vietnam - Ministry of Health or sent by post. Within 3 working days from the date of receipt of valid dossiers and publication fees, the Drug Administration of Vietnam will issue cosmetic publication receipt numbers to individuals and businesses.
3. Customs procedures when importing cosmetics
After receiving the cosmetic proclamation receipt number, individuals and businesses carry out customs procedures to import goods into Vietnam. Individuals and businesses need to take the following steps:
- Prepare customs documents including the following papers and documents:
- Customs declaration of imported goods (according to form No. 01, Appendix II issued with Circular 39/2018/TT-BTC) or documents replacing the customs declaration.
- Commercial documents or invoices have equivalent value in case the buyer pays the seller.
- Transport documents or bills of lading have equivalent value in the case of products transported by air/rail/sea/multimodal transport.
- Document or import license.
- Certificate of specialized inspection.
- Types of documents proving that individuals/organizations are eligible to import goods.
- Value declaration.
- Entrustment contract and certificate of origin (C/O).
- Valid receipt number of imported cosmetic declaration form.
- Submit customs documents at the border gate where goods enter Vietnam via electronic customs software or directly at the competent customs authority. The deadline for submitting documents is before the date the goods arrive at the border gate or within 30 days from the date the goods arrive at the border gate.
- Pay taxes and import fees according to regulations. Import tax is calculated based on the dutiable value of the goods and the applicable tax rate. The import fee is 0.2% of the dutiable value of the goods.
- Receive customs clearance permit and pick up goods. During the customs clearance process, the customs authority may request a physical inspection of the goods to compare with the declared information and cosmetic proclamation receipt number.
Some related articles:
- Cosmetic Jars
- Importing Chinese clothes into Vietnam: Challenges and opportunities in the fashion industry
These are the basic steps to import cosmetics into Vietnam. Depending on the type of cosmetics and quantity imported, there may be different requirements for documentation and procedures. Therefore, individuals and businesses should carefully study the relevant regulations and seek advice from experts or import service companies to implement them smoothly and effectively.
HBS Vietnam has been supporting and consulting more than 1,000 businesses across the country in many different industries, from agricultural products, seafood, household appliances to production machinery and many other industries. Please contact HBS Vietnam today for more detailed information and to receive attractive offers.
- HBS Vietnam is the official authorized agent of Alibaba in Vietnam.
- Service support hotline: 0243 626 2288 – 1900 25 25 89
- Zalo/SMS: 0938 11 6869
- Email: info@hbsvietnam.com
- Website: https://hbsvietnam.com/
- Office: No. 69 Le Duc Tho, My Dinh 2, Nam Tu Liem, Hanoi
- Ho Chi Minh City Office: No. 6 Nguyen Trung Truc, Ward 5, Binh Thanh, Ho Chi Minh
Hopefully this article has provided you with useful information about the latest updated cosmetic import procedures. If you have any questions or comments, please contact us. Thank you for your interest!